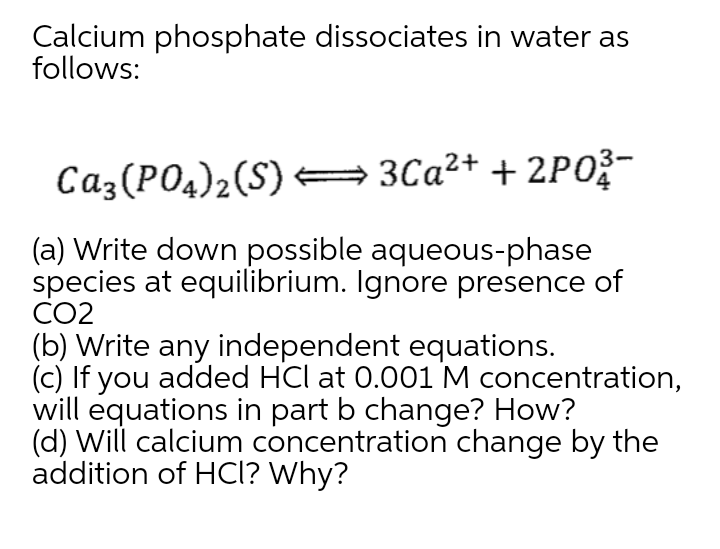
Calcium Phosphate Dissociation Equation . write net ionic equations for chemical reactions between ionic compounds. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. The calcium nitrate formula unit. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. the solubility of calcium salts is highly dependent on ph p h. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. The chemical reaction is given. This is because of the 2+ 2 + charge of the calcium ion. For example, let’s look at tricalcium phosphate, which presents. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically.
from www.bartleby.com
The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. The calcium nitrate formula unit. For example, let’s look at tricalcium phosphate, which presents. the solubility of calcium salts is highly dependent on ph p h. The chemical reaction is given. This is because of the 2+ 2 + charge of the calcium ion. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. write net ionic equations for chemical reactions between ionic compounds. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×.
Answered Calcium phosphate dissociates in water… bartleby
Calcium Phosphate Dissociation Equation Calcium phosphide reacts with water to form phosphane and calcium hydroxide. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. the solubility of calcium salts is highly dependent on ph p h. For example, let’s look at tricalcium phosphate, which presents. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. write net ionic equations for chemical reactions between ionic compounds. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. The calcium nitrate formula unit. This is because of the 2+ 2 + charge of the calcium ion. The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. The chemical reaction is given.
From www.researchgate.net
Phosphate dissociation from actoTmTnmyosinS1A1ADPPi at high calcium Calcium Phosphate Dissociation Equation [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. The chemical reaction is given. This is because of the 2+ 2 + charge of the calcium ion. For example, let’s look at tricalcium phosphate, which presents. Two nitrate ions, each with a 1− 1 − charge are. Calcium Phosphate Dissociation Equation.
From www.nutrihairun.com
calcium phosphate Calcium Phosphate Dissociation Equation write net ionic equations for chemical reactions between ionic compounds. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. This is because of the 2+ 2 + charge of the calcium ion. The ammonium phosphate formula unit dissociates into three ammonium ions. Calcium Phosphate Dissociation Equation.
From www.researchgate.net
Phosphate dissociation from actoTmTnmyosinS1A1ADPPi at low calcium Calcium Phosphate Dissociation Equation For example, let’s look at tricalcium phosphate, which presents. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. the solubility of calcium salts is highly dependent on ph p h. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. The ammonium phosphate formula unit dissociates into three ammonium ions. Calcium Phosphate Dissociation Equation.
From learnmedicine.org
Calcium and Phosphate Regulation LearnMedicine Calcium Phosphate Dissociation Equation Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. This is because of the 2+ 2 + charge of the calcium ion. The calcium nitrate formula unit. write net ionic equations for chemical reactions between ionic compounds. The chemical reaction is given. in this video we will describe the. Calcium Phosphate Dissociation Equation.
From www.bartleby.com
Answered Calcium phosphate dissociates in water… bartleby Calcium Phosphate Dissociation Equation The calcium nitrate formula unit. For example, let’s look at tricalcium phosphate, which presents. the solubility of calcium salts is highly dependent on ph p h. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. The ammonium phosphate formula unit dissociates into three ammonium ions and. Calcium Phosphate Dissociation Equation.
From formulasense.com
What is Calcium Phosphate? — Formula Sense Calcium Phosphate Dissociation Equation the solubility of calcium salts is highly dependent on ph p h. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. . Calcium Phosphate Dissociation Equation.
From www.researchgate.net
Phosphate dissociation from actoTmTnmyosinS1A1ADPPi at low calcium Calcium Phosphate Dissociation Equation The chemical reaction is given. the solubility of calcium salts is highly dependent on ph p h. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. Calcium phosphide reacts with water to form phosphane and calcium. Calcium Phosphate Dissociation Equation.
From brainly.in
Write the steps involved in writing the chemical formula of calcium Calcium Phosphate Dissociation Equation The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the 2+ 2 + charge of the calcium ion. in this video we will describe. Calcium Phosphate Dissociation Equation.
From brainly.in
Predict what happens when a potassium phosphate solution is mixed with Calcium Phosphate Dissociation Equation For example, let’s look at tricalcium phosphate, which presents. The chemical reaction is given. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. This is because of the 2+. Calcium Phosphate Dissociation Equation.
From www.youtube.com
Writing the Formula for Calcium Phosphate YouTube Calcium Phosphate Dissociation Equation Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. The calcium nitrate formula unit. in this video we will describe the equation ca3 (po4)2 + h2o and write. Calcium Phosphate Dissociation Equation.
From www.numerade.com
SOLVED The Ksp value for Calcium Phosphate [Ca3(PO4)2] is 1.00 x 1026 Calcium Phosphate Dissociation Equation in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. For example, let’s look at tricalcium phosphate, which presents. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. the calcium nitrate formula unit dissociates into one calcium ion and. Calcium Phosphate Dissociation Equation.
From ceaqrhjq.blob.core.windows.net
Zinc Iron Iii Chloride Balanced Equation at Carolyn Smith blog Calcium Phosphate Dissociation Equation [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. The ammonium phosphate formula unit dissociates into three ammonium ions and one phosphate ion. write net ionic equations for chemical reactions between ionic compounds. the solubility of calcium salts is highly dependent on ph p h. the calcium nitrate formula unit dissociates into one calcium ion. Calcium Phosphate Dissociation Equation.
From nikolasfersirwin.blogspot.com
Write the Formula for the Dihydrogen Phosphate Ion. Calcium Phosphate Dissociation Equation the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. The calcium nitrate formula unit. the solubility of calcium salts is highly dependent on ph p h. For example, let’s look at. Calcium Phosphate Dissociation Equation.
From www.researchgate.net
Phosphate dissociation from actoTmTnmyosinS1A1ADPPi at low calcium Calcium Phosphate Dissociation Equation The calcium nitrate formula unit. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. For example, let’s look at tricalcium phosphate, which presents. the solubility of calcium salts is highly dependent on ph p h. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. mpxq(s) ⇌ pmm+(aq). Calcium Phosphate Dissociation Equation.
From www.wikilectures.eu
Disorders of Calcium And Phosphate Metabolism WikiLectures Calcium Phosphate Dissociation Equation the solubility of calcium salts is highly dependent on ph p h. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. Calcium phosphide reacts with water to form phosphane and calcium hydroxide. write net ionic equations for chemical reactions between ionic compounds. The calcium nitrate formula unit. This is. Calcium Phosphate Dissociation Equation.
From www.chegg.com
Solved The K_sp of calcium phosphate is 2.0 times 10^29 at Calcium Phosphate Dissociation Equation Calcium phosphide reacts with water to form phosphane and calcium hydroxide. For example, let’s look at tricalcium phosphate, which presents. The chemical reaction is given. Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. mpxq(s) ⇌ pmm+(aq) + qxn−(aq) caf2(s) ⇌ ca2+(aq) +2f−(aq) ×. the solubility of calcium salts. Calcium Phosphate Dissociation Equation.
From www.youtube.com
How to Write the for Equation for Ca3(PO4)2 + H2O (Calcium phosphate Calcium Phosphate Dissociation Equation For example, let’s look at tricalcium phosphate, which presents. [f−] =(2molf− /1molca2+) = (2)(2.15 × 10−4m) = 4.30 × 10−4m. the solubility of calcium salts is highly dependent on ph p h. write net ionic equations for chemical reactions between ionic compounds. The chemical reaction is given. the calcium nitrate formula unit dissociates into one calcium ion. Calcium Phosphate Dissociation Equation.
From www.numerade.com
SOLVED Write dissociation reactions of the following ionic compounds Calcium Phosphate Dissociation Equation Two nitrate ions, each with a 1− 1 − charge are required to make the equation balance electrically. in this video we will describe the equation ca3 (po4)2 + h2o and write what happens. the calcium nitrate formula unit dissociates into one calcium ion and two nitrate ions. the solubility of calcium salts is highly dependent on. Calcium Phosphate Dissociation Equation.